
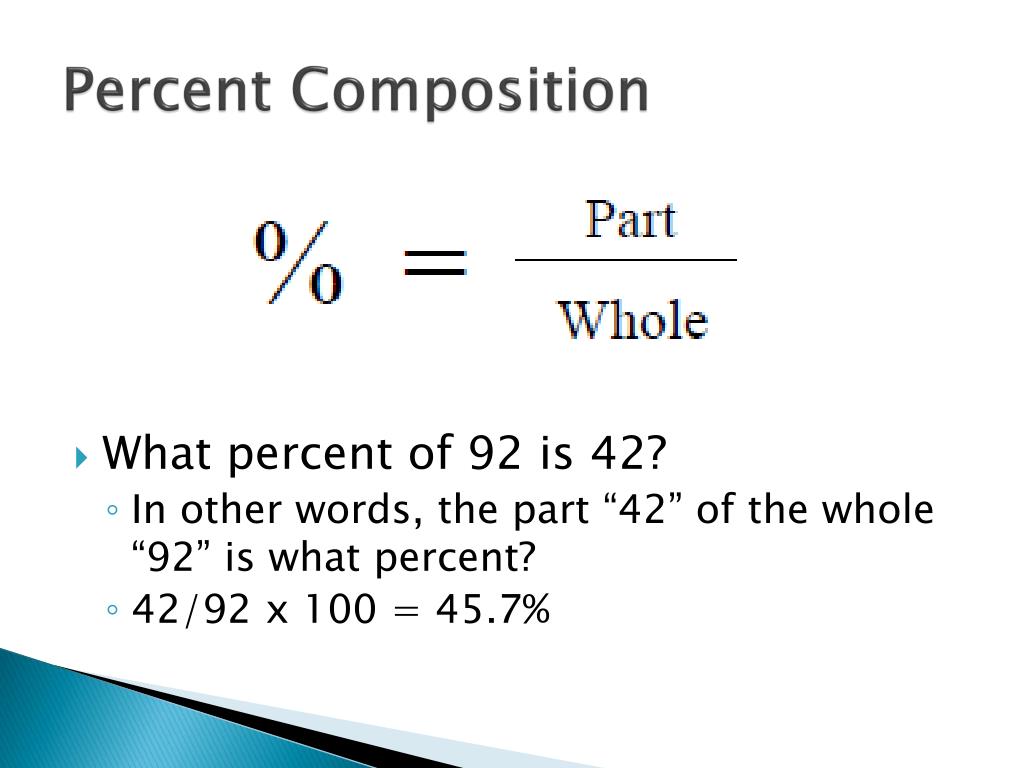
To express the value as a percentage, you need to multiply by 100 at the top. Mass percent (mass of chemical / total mass of compound) x 100 is the formula for the mass percent of a compound. Step 1: Define a formula for calculating a compound’s mass percent. If the number is too far to round (x.1 ~ x.9), then multiply each solution by the sameįactor to get the lowest whole number multiple. Finding the Mass Percentage in Simple Steps. Calculate the total mass of the compound. percent v/v solution is calculated by the following formula using the milliliter as the base measure of volume (v): v/v mL of solute/100 mL of solution. Mass Mass of solute Mass of solution Mass of solute Mass of solution x 100. To specify the value as a percentage, multiply the top by 100.
This is the mole ratio of the elements and is represented by subscripts in the empirical formula. The basic formula for the mass percentage of a compound is mass percentage (mass chemicals total mass compound) x 100. The mass of each element = the percent given.Ĭonvert the mass of each element to moles using the molar mass from the periodic table.ĭivide each mole value by the smallest number of moles calculated. If percentages are given, assume that the total mass is 100 grams so that Start with the number of grams of each element, given in the problem.

(The molar mass of NutraSweet is 294.30 g/mol) Calculate the empirical formula of NutraSweet and find the molecular formula. The comparison is done in the form of masses, not by the volume. Although mass percent is unit-less it can be given with units as well in special cases. The mass percentage is just an excellent style for presenting the concentration of an element within a compound or component of the mixture. Once we have this information we can convert it to moles to determine the ratios between the elements.Ī simple rhyme can be used to remember the process: The mass percent formula in Chemistry is given as. In order to determine the Empirical formula for a compound or molecule, we need to know the mass percentages of the the elements in the compound. This would be the lowest whole number ratio of the elements in the compound. The Empirical FormulaĪn Empirical formula is the chemical formula of a compound that gives the proportions (ratios) of the elements present in the compound but not the actual numbers or arrangement of atoms. In this lecture we cover the relationship between Empirical and Molecular formulas and the calculations used to determine one from the other. Therefore, the mass% of NaCl in solution is 20%.The content that follows is the substance of lecture 9. Mass% of NaCl in this solution = (25 g / 125 g) 100% = 20%
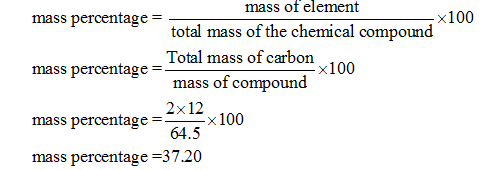
Therefore, solution mass = solute Mass + mass of solvent = 100 g + 25 g = 125 g Percentage of mass (solute’s mass ÷ mass of solution) x 100. Solvent mass (deionized water) = 100 g (1 ml deionized water weighs about 1 g) Mass percent (component’s mass ÷ total mass) x 100 or. Percentage of marks obtained means the total obtained by the student considering the fact had the total been 100 what would be his score. Q2: Determine the percentage of a solution containing 25.0 g NaCl dissolved in 100 mL deionized water. Answer: Percentages are worked for two quantities of the same kind, not between two different items. Enter a valid molecular formula and press the calculate button to determine. The formula for mass per cent is provided below: Mass per cent (mass of component/total mass) x 100. In addition, any amount of NaOH can be dissolved in deionized water in a 3:7 ratio to form a 30% NaOH solution. Determine the Molar mass and Percent mass composition of a linear chemical formula.
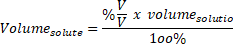
A 30% solution of NaOH in water should be produced. Therefore, dissolve 30g of NaOH in 70g of deionized water. X g of solute in 100g of solution = 30.00.0g of NaOH = X Substitute the value of the weight percent known in the basic formula Q1: Calculate the number of grams of NaOH needed to make a 30.0% solution using deionized water as the solvent. Per cent by weight = gram of solute/100 g of solution Solved Examples
#Formula for percent by mmass plus
To determine the weight percent of a solution, divide the mass of the solute by the mass of the solution (the solute plus the solvent) and multiply by 100 to get the percentage. A solution may be expressed as a relative percentage concentration of solute in the solution. In addition to molarity, normality, or molality, the solution can also be described in other concentrations.


 0 kommentar(er)
0 kommentar(er)
